The technology needed to build a clean, affordable and sustainable energy storage system anywhere.

Head of the University of Bristol Electrochemistry and Solar Team, observed:
Revolutionary Hydrogel Based Storage
We face a global challenge – to create an affordable new energy system, the cost of intermittent renewable electricity generation plus energy storage, must be below the cost of fossil fuel derived electricity. Solar electricity is now less than half the cost of fossil fuels. The bottleneck is low cost and safe energy storage technology.
Our revolutionary hydrogel-based storage is being commercialised to address this challenge. This breakthrough could significantly lower electricity bills and play an essential role in reducing fuel poverty.
We have developed and patented hydrogel membrane technologies that enable new rapid-to-charge and safe electricity storage solutions. The technology is low cost and uses abundant and widely available raw materials which offer a secure supply chain.
A closer look
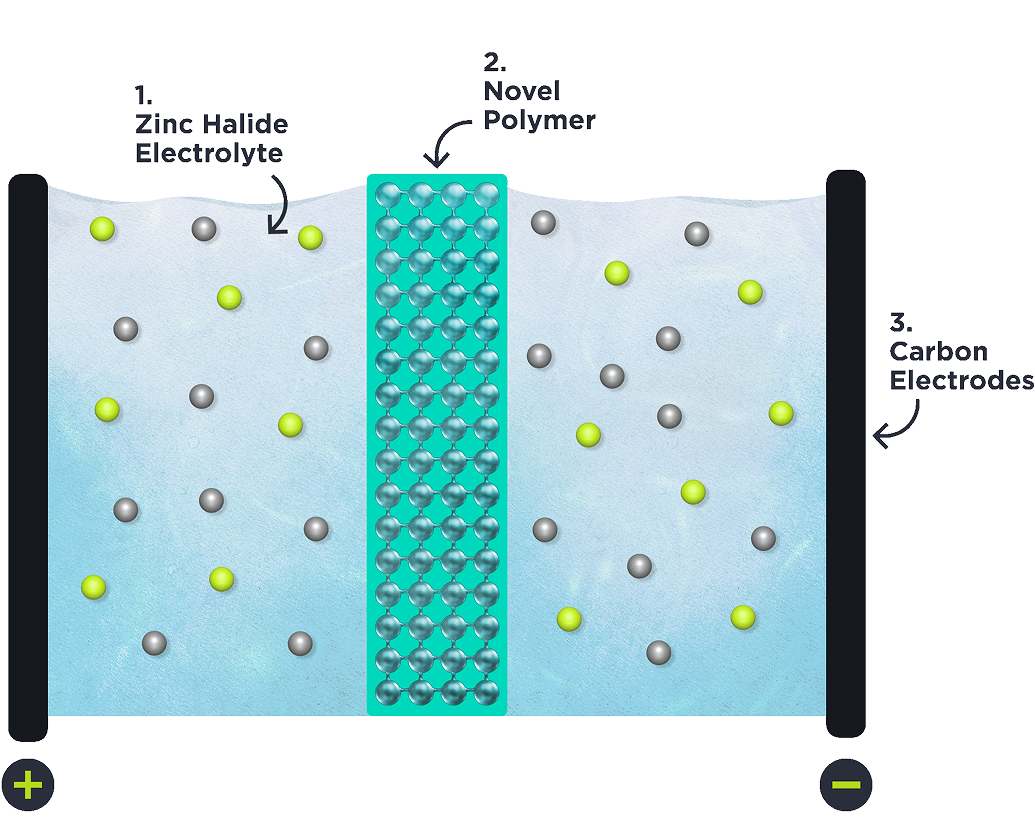
1. Zinc Halide Electrolyte
Water-based Zinc Halide electrolyte energy storage systems are over 100 years old and have a high theoretical energy storage capacity that exceeds standard batteries.
However, these systems have seen limited commercialisation because stable performance is either reliant upon expensive membranes or slow reacting chemical additives which significantly reduce charge rates.
Our polymer breakthrough overcomes these limitations. This breakthrough enables a high-performance system featuring both chemical battery storage and electrostatic supercapacitor storage.
2. Novel Polymer
Our novel polymer membrane is ion selective, allowing certain favourable ions to pass while preventing crossover of other, non-favourable, ions contributing to stable performance and long life. In addition, this membrane has high ionic conductivity: enabling rapid transference of favourable ions, facilitating rapid charge times.
Our polymer is low cost, being approximately two orders of magnitude cheaper to manufacture than commercially available ion selective membranes.
3. Carbon Electrodes
Both the anode and cathode are made of activated carbon.
Key Advantages of Our Technology








